Defense of MOCA during TSCA Review
The TSCA review process has an estimated duration of an additional 5 years, finishing in Q2, 2030. Following extensive monitoring, review and involvement, the PMA Leadership has identified a path forward to defend MOCA. This strategy has an estimated cost of approximately $2 million.
How to Get Involved
- Click here to contribute funds towards MOCA defense.
- Provide PMA access to your Toxicologist. (Click here to email contact info.)
- Send a letter to your legislators in support of this strategy.
- Click here to download the template letter. (coming soon)
- Click here to locate your legislators.
- Volunteer to be involved with the Government Affairs Committee.
- Spread the word! Share this information with any members, non-members, suppliers and anyone else that is willing to support the defense of MOCA.
Next Steps
- Obtain funds to support MOCA defense.
- Find 2 experienced EHS personnel (e.g., air, waste, water experience) and 2-3 R&D heads with TSCA PMN experience.
- Find a toxicologist either within PMA or externally with prior TSCA Existing Chemical Review experience.
TSCA Existing Chemical Review Overview
(information shared at the 2025 PMA Annual Meeting)
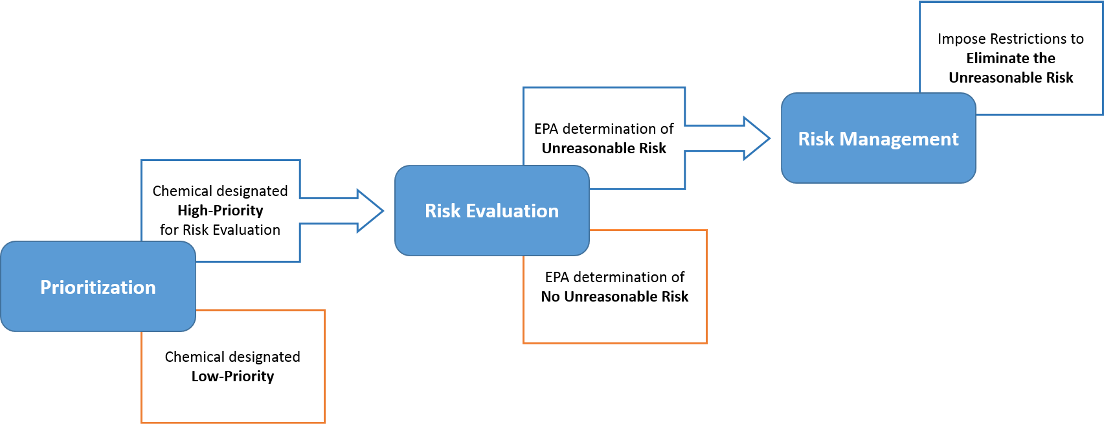
Prioritization
1 year process in which EPA determines if a chemical is a High Priority or Low Priority Substance (offramp to review).
- MOCA started Initiation of Prioritization on 12/18/23.
- PMA utilized the rifle shot and load the record approach.
- The rifle shot presented the case that MOCA did not belong in the TSCA review process and should be a Low Priority Substance.
- PMA met with EPA to review the rifle shot.
- The load the record approach consisted of submitting 3000 pages of documents to EPA to build the case for future litigation if desired.
- Prioritization ended on 12/18/24 with MOCA being listed as a High Priority Substance.
Risk Evaluation
3 - 3.5 year process in which EPA determines whether the condition(s) of use (1st Trump Admin) or “whole chemical” (Biden Admin) poses an unreasonable risk to the human health or the environment.
- Risk Evaluation started on 12/18/24.
- EPA published preliminary payee list on 12/31/24.
- Public comment was held through 3/3/25.
- Final list will be published no later than concurrent with publication of the final risk evaluation scope document.
- The entire review process for MOCA through Risk Management is expected to be complete in 2030.
- One goal would be to try to complete Risk Management before the 2nd Trump Administration ends.
Risk Management
2 year process in which EPA will ultimately regulate the chemical until it no longer poses an unreasonable risk.
- EPA is given a range of risk management options under TSCA, including labeling, recordkeeping or notice requirements, actions to reduce human exposure or environmental release, and a ban of the chemical or of certain uses.
What Does Success Look Like For MOCA
- At the end of Risk Management, PMA MOCA Safe Handling Guidelines is written into the requirements.
- No bans or restrictions beyond PMA MOCA Safe Handling Guidelines.
|


